How Antibiotic Resistance Became an Urgent Threat
Until the 1940s, when penicillin became the first widely available antibiotic, the odds of surviving many bacterial infections were poor. It wasn’t unusual to die of strep throat or become deaf as a result of an ear infection. No effective treatment existed for illnesses such as gonorrhea, bacterial pneumonia, rheumatic fever and other infectious diseases.
Before penicillin, even a simple scratch on the knee could have devastating consequences.
Discovered accidentally by Alexander Fleming in 1928, penicillin was hailed as a “wonder drug” and gave the world hope that innumerable infectious diseases could be cured once and for all. Further research and development armed doctors with many powerful antibiotics engineered to either kill bacteria or prevent their cell walls from multiplying. This powerful arsenal has reduced the number of deaths linked to bacterial illness, raised life expectancy and saved millions of lives.
The discovery of penicillin also launched a medical revolution, paving the way for preventing or treating infections in patients who receive chemotherapy, suffer from chronic diseases, or undergo organ transplants and other complex surgeries.
Changing Tides in Antibiotic Use
Although the “era of antibiotics” began with great promise, it didn’t take long for the laws of natural selection to tamper with the efficacy of the wonder drugs. Bacteria-battling treatments were outsmarted by the microbes they were meant to destroy. Under continuous assault from antibiotics, bacteria, by the untold billions, fought back, mutating or swapping genes with other organisms in order to dodge extinction.
Hundreds of millions of courses of antibiotics are prescribed every year in the United States. Additionally, antibiotics are extensively overused in agriculture. While there are circumstances where antibiotics are necessary in food animal production (i.e., to treat sick animals), the vast majority of these vital drugs are used to promote growth and prevent illness. In the U.S. alone, 70 percent of all antibiotics of medical importance to humans are sold for use in food animals. This rampant overuse in agriculture also contributes to the rise of antibiotic-resistant bacteria that can spread to humans and animals.
Overuse of antibiotics promotes resistance by leaving behind genetically agile bacterial strains able to survive treatment, quickly multiply and spread from individuals to family, friends and anyone they come in contact with. Antibiotic resistance in bacteria occurs in several ways. The laws of natural selection ensure that some bacteria will continue to survive exposure to antibiotics from generation to generation. Other bacteria can develop mechanisms for resisting drugs through biological mutation. It is also possible for bacteria to exchange antibiotic-resistant DNA.
Bacteria responsible for a wide range of infections have evolved into drug-resistant “superbugs,” putting millions of lives at risk. According to the Centers for Disease Control and Prevention (CDC), at least 2 million people in the United States become infected with antibiotic-resistant bacteria each year, and some 23,000 people die annually from these infections. By CDC’s own admission, these are very conservative estimates.
The emergence of superbugs has alarmed public health officials worldwide who fear that antibiotics may ultimately become useless. The World Health Organization puts the situation in stark terms: “Without urgent, coordinated action, the world is heading toward a post-antibiotic era, in which common infections and minor injuries, which have been treatable for decades, can once again kill.”
The Threat of Resistance
In the United States, hospitals and other medical settings have become particularly lethal breeding grounds for antibiotic-resistant bacteria, imperiling vulnerable patients who contract health-care-associated infections (HAIs). Public health experts warn that common HAIs, such as pneumonia, cystitis, staph and bloodstream infections, will no longer respond to antibiotics if those antibiotics are not used and prescribed judiciously; nor will it remain possible to prevent or treat infections linked to surgery or effectively control infections in neonatal and intensive care units.
Of especially grave concern are bacteria such as CRE (carbapenem-resistant Enterobacteriaceae), a highly resistant family of bacteria that can cause difficult-to-treat and frequently deadly infections, according to a report cited by the CDC. In 2011, a CRE outbreak at a National Institutes of Health clinic infected 18 patients and killed 11.
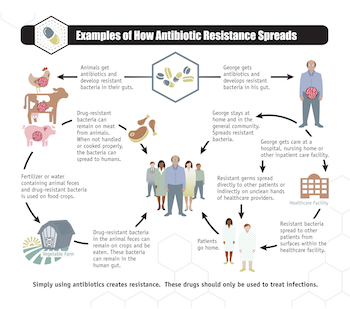
As resistant bacteria continue to proliferate, dozens of diseases are becoming more difficult to treat with antibiotics. In many regions, the number of resistant cases of gonorrhea, dysentery, multidrug resistant tuberculosis and other respiratory diseases has reached troubling levels. Although last-resort drugs have been effective in treating drug-resistant infections, they tend to be very expensive and scarce. Even more disturbing is the discovery of bacteria with a dangerous antibiotic-resistance gene, mcr-1, that bestows resistance to the antibiotic colistin, which is considered the last drug available to treat some deadly infections.
There is strong evidence that this gene evolved due to overuse of colistin in food animal production. This gene has been found in people, food animals, food products and pets in the U.S. and in least 30 countries around the world since November 2015.
The Future of Antibiotic Use
Even when used effectively, every course of antibiotics increases the survival rate for selected bacteria that are not susceptible to medication. Blame for the explosion of antibiotic-resistant organisms rests on various culprits, including the excessive unnecessary use of antibiotics in agriculture, the large number of needless prescriptions written by health care providers and the misuse of the antibiotics by patients.
A recent report released by the CDC, in collaboration with The Pew Charitable Trusts and other experts, found that 30 percent of antibiotics prescribed in outpatient settings in the United States are unnecessary. Most of these prescriptions are written for viral illnesses, such as colds, sore throats and flu, that don’t respond to antibiotics. The study also attributes the spread of drug resistance to health care providers who prescribe antibiotics incorrectly: at the wrong dose or for the incorrect length of time.
Patients who misuse antibiotics encourage the spread of resistance as well. An incomplete course of prescribed antibiotics is an invitation for harmful bacteria to develop resistance. So is skipping a dose. Likewise, sharing drugs or using leftover prescriptions gives a boost to resistant bacteria. Without treatment, a skin infection or surgical wound that doesn’t get better or worsens can also empower bacteria to strengthen and spread.
Antibiotic resistance has been described as one of the greatest public health threats of our time. Understanding the dangers of antibiotic resistance is a given for public health students. Educating the public is essential as well. Initiatives, such as the Antibiotic Resistance Action Center, housed in the Milken Institute School of Public Health at the George Washington University, are lending their expertise to further scientific research on this global health crisis, while promoting public awareness and understanding and a path forward to help reduce antibiotic resistance worldwide.
Health care providers, patients, families, the agriculture sector and policymakers must all take responsibility for sustainable use of antibiotics. These drugs are not a cure-all. And, when used irresponsibly and unnecessarily, antibiotics hasten the spread of deadly antibiotic-resistant bacteria. The future of global health is on the line, as every single antibiotic drug course triggers a bacterial response that extends to every corner of the world. This ripple effect threatens to return us to a pre-antibiotic world, where illnesses that are curable today again become fatal.


